When it comes to pharmaceutical manufacturing, ensuring a strong quality culture is one way to avoid recurring GMP issues, such as data integrity. Susan Schniepp, Distinguished Fellow at Regulatory Compliance Associates, and 2022 PDA Board of Directors Chair outlined the evolution of current quality culture initiatives and showcased some tools to help organizations build quality culture into GMP manufacturing operations during the Nov. 11, 2021, Redica Systems Quality Week webinar, “Past, Present, and Future of Quality Culture.”
“I think that is what we see sometimes in our industry,” she said. “We do not remember how we got some of the regulations that exist today, and we repeat our past mistakes.”
From Quality Metrics to Quality Culture
Schniepp began her presentation with a look at how quality culture evolved from the early 2010s discussion on quality metrics. Key points included the following:
- On Oct. 5, 2005, CDER Director Janet Woodcock directed pharma manufacturers to build “a maximally efficient, agile, flexible pharmaceutical manufacturing sector that reliably produces high quality drug products without extensive regulatory oversight.” This set in motion CDER’s emphasis on quality in manufacturing.
- Then, in 2012, the U.S. Congress passed the FDA Safety and Innovation Act (FDASIA). Title VII of the Act allows for risk-based inspection of manufacturing sites, including requesting records in advance, or in lieu of an inspection. Additionally, the Act requires the FDA to provide Congress with an annual report on the state of drug shortages.
- Around the same time that FDASIA became effective, drug shortages became a critical concern for the agency. Schniepp pointed to aging facilities as one reason for shortages. “Tangential to metrics and quality culture, we had aging facilities, i.e., facilities producing many of the drugs showing up on the drug shortage list were degrading and not being maintained.” Quality issues at these sites had become apparent and potentially causing shortages.
- In 2014, FDA proposed implementation of a quality metrics system to encourage adoption of quality-supporting practices.
GMP Manufacturing & Measuring Quality Culture
The quality metrics push resulted in a series of conferences by ISPE, PDA, and the Brookings Institution (Figure 1 below).
“One of the elements that came out of all of these discussions was the fact that you could request any metrics that you wanted. What FDA proposed initially was rejection rate on batches, CAPA rates, etc., but the conversation became that without the culture of the organization being assessed, you could not really count on the data. So, that is how [quality metrics and quality culture] tie together,” Schniepp explained.
Quality Metrics
“You needed to have a functioning, efficient, quality metric system that gives you reliable data. And you have to count on the integrity of the culture of an organization in order to have confidence in the data that it sends you. Off of that, the discussion turned to, ‘well, how do you measure quality culture?’”
GMP Manufacturing & Quality Culture Behavior
Schniepp was part of a PDA team studying the relationship between quality culture behavior and mature quality attributes to see if there was a set of mature quality attributes that could serve as a surrogate for quality culture behaviors (ISPE has also done work in this area).
“’Mature quality attribute’” refers to “those things in your quality management system (QMS) such as your CAPA program, your change control, your investigations procedures, how you report deviations, how you record batch rejection rates, i.e., all of those things are in your quality culture or in your QMS.”
Quality Attributes
But her team wanted to know if mature quality attributes related to the behaviors of employees and management correlated with company culture, such as do people feel comfortable speaking up, do they talk to management about their concerns, do they understand their jobs, etc. The team’s research resulted in what Schniepp refers to as “the Culture Equation” (Figure 2).
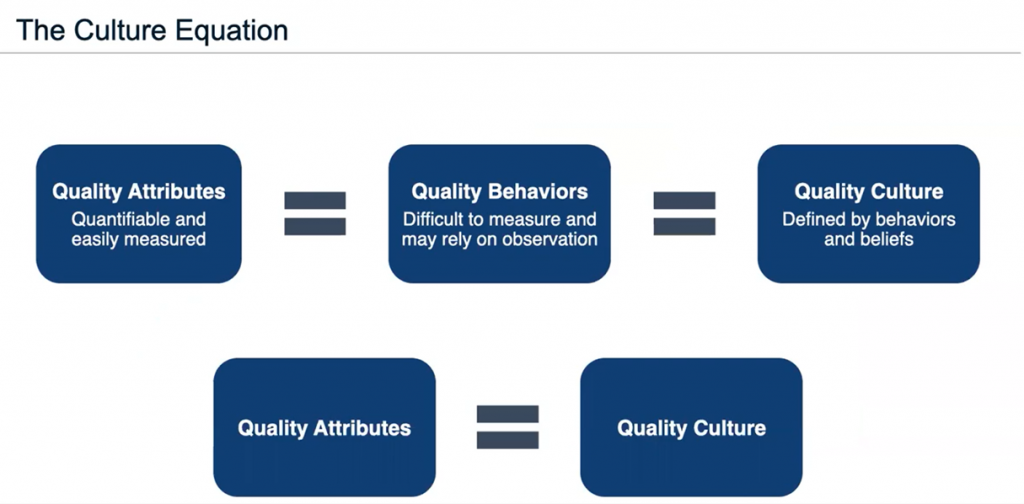
“The foundation of this is quality attributes that are quantifiable and easily measured, provided they equal quality behaviors,” she said. “In other words, you have attributes reflected in your behaviors that then define your quality culture. So, if quality cultures equal quality behaviors equals quality culture, your quality attributes equal quality behaviors equal quality culture. If you can measure your quality attributes, you can define your quality culture.”
Operational Excellence
By surveying PDA’s membership, her team gathered enough data to conduct statistical analysis and was able to determine there is a relationship between quality culture behavior and quality attributes. They then worked with another team from the University of Saint Gallen’s operational excellence program. That team also found a correlation confirming “quality attributes equal quality behaviors equal quality culture.”
The PDA and St. Gallen research resulted in the PDA Quality Culture Guided Assessment Tool, addressing five key areas in which companies should focus in order to build quality culture: Employee Ownership and Engagement, Continuous Improvement, Technical Excellence, Leadership Commitment, and Communication and Collaboration. This tool is currently under development as a PDA standard.
Quality Metrics, Data Integrity & Quality Culture
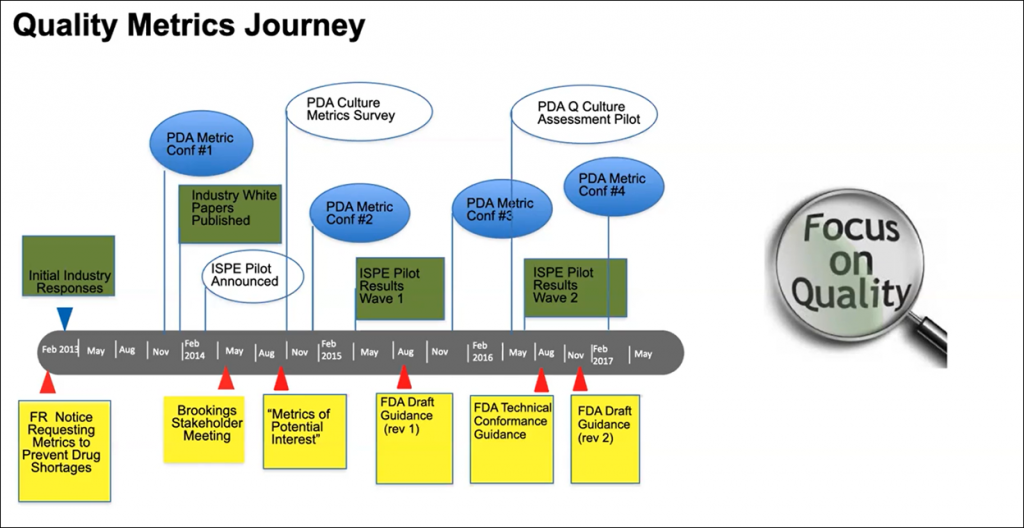
Figure 1
Data integrity is increasingly connected with quality culture. In the PIC/S guidance, Good Practices For Data Management And Integrity in Regulated GMP/GDP Environments, “they have defined quality culture, and they have given us a little bit of information about how management can create a work environment—a culture—that is transparent and open.”
Schniepp also pointed to three other documents:
- UK MHRA GxP Data Integrity Definitions and Guidance for Industry
- WHO Guidance on Good Data and Record Management Practices
- FDA guidance Data Integrity and Compliance With Drug CGMP Questions and Answers
“In their regulations, regulators are starting to define and link culture to data integrity violations,” she explained.
Data Integrity
She then provided a real-life example of a data integrity violation caused by a company’s culture.
“This one is kind of an interesting one because it was really an attempt by management to encourage employees to do a good job, but what they did created integrity violations.”
A contract manufacturing organization (CMO) set a GMP manufacturing goal to release batch records to clients within 30 days of manufacturing the product. To encourage this, management dangled the carrot of a pizza party at the end of the year if this goal was met. In order to meet this goal, employees released batch records to clients even if investigations had not uncovered the root cause. Investigations would be prematurely closed and then reopened after the batch record had been sent to the client.
“That, in and of itself, is a data integrity nightmare,” Schniepp said. “Can you imagine explaining in an audit by a regulator, ‘well, we wanted a pizza party?’”
GMP Manufacturing & Organizational Culture
Regarding deviation investigations, she told another story of a company for whom she worked that involved repeat observations. When reviewing deviations, she found that one operator had 30 deviations tied to him involving a specific product. This operator failed to sign the batch record during a specific point in time in manufacturing. His managers kept retraining him.
[Author’s Note: Additional information about deviation investigations can be found in the articles, “GMP Inspection Case Study Focuses on Inadequate Deviation Investigations” and “How to Avoid Three Common Deviation Investigation Pitfalls.”]
Batch Records
In talking with the operator, Schniepp learned that in order for the operator to sign the batch record (according to GMP regulations at that particular point in manufacturing), the operator had to leave the product alone in the equipment, de-gown, sign the batch record, re-gown, and return, all the while hoping the product was okay. Out of concern for the product, the operator chose to stay behind and try to remember to sign the batch record once he was done.
“It was a poorly designed batch record process,” she said. But the operator did not feel listened to when he brought his concerns to management.
GMP Labeling
Schniepp then pointed out that other employees worked on the product under the same cGMP constraints. So, what were these other employees doing? How many other data integrity violations occurred? Was GMP labelling reviewed accurately?
“Nobody else was bringing it up. If you do not have that open culture, if you are not encouraging people to speak up and you are not listening to them, you do not have a good quality culture.”
The Future is GMP Manufacturing Quality
For further evidence of the importance of GMP certification and quality culture, Schniepp reviewed a 483 observation issued in 2019 with numerous examples of data mismanagement. The company in question then responded to FDA with a letter promising to ensure a robust quality culture going forward. [Author’s Note: For more about this GMP compliance incident, read the first case study in the article, “Data Integrity Concerns Discovered in Gene Therapy Product Submissions,” by Jerry Chapman.]
FDA 483
Schniepp believes this 483 observation shows FDA’s intent to heavily focus more on good manufacturing practices in future inspections. This means companies must develop or enhance their quality culture when thinking about how to prepare for upcoming inspections.
“I believe we will see more of this,” she said. “More companies will be looking at their GMP manufacturing and trying to improve the Quality Culture.”
Otherwise, as she explained at the beginning of her presentation, companies who remain in the past when it comes to cGMP certified practices that lead to quality culture face the prospect of 483 observations, Warning Letters, and other negative outcomes.
About RCA’s Pharmaceutical Consulting Services
Regulatory Compliance Associates (RCA) has helped thousands of pharmaceutical companies meet regulatory, compliance, quality assurance, and remediation challenges. With more than 20 years of experience with FDA, Health Canada, EU and global regulatory agencies worldwide, Regulatory Compliance Associates® offers leading pharmaceutical consultants. We’re one of the few pharma consulting companies that can help you navigate the challenges associated with industry regulations.
Our pharmaceutical consulting firm includes over 500 seasoned FDA, Health Canada & EU compliance consultants and regulatory affairs experts who understand industry complexities. It’s a pharma consultancy founded by regulatory compliance executives from the pharmaceutical industry. Every pharmaceutical industry consultant on the Regulatory Compliance Associates team knows the unique inner workings of the regulatory process.
Client Solutions
Whether you’re in the product planning, development or pharmaceutical lifecycle management stage or need a remediation strategy for a compliance crisis, Regulatory Compliance Associates will guide you through every pharmaceutical consulting step of the regulatory process and create a customized approach depending on your product and your pharma company’s individual needs. Our regulatory compliance clients include:
- Companies new to FDA, Health Canada or EU regulations and regulatory compliance
- Start-up organizations with novel submissions to 510(k) submissions from multi-national corporations
- Investment firms seeking private equity due diligence for pre-acquisition and post-deal research
- Law firms seeking pharmaceutical consulting firm expertise in the remediation of warning letters, consent decrees, 483’s or import bans
Regulatory Affairs
Regulatory affairs is Regulatory Compliance Associates backbone. We exceed other pharma consulting companies with industry experts experienced in complexities of the pharmaceutical and biopharmaceutical industries. Our pharma consulting expertise spans all facets and levels of Regulatory Affairs, from Regulatory Support for New Products to Life Cycle Management, to other services like Outsourced Regulatory Affairs, Submissions, Training, and more.
As your partner, we can negotiate the potential assessment minefield of regulatory compliance services with insight, hindsight, and the clear advantage of our breadth and depth of knowledge and regulatory compliance consulting. We offer the following pharma consulting regulatory affairs services for pharmaceutical companies.
- New Product Support
- Product Lifecycle
- Other Regulatory Services
- Combination Products
Compliance Assurance
The regulations process surrounding pharmaceutical companies can be tricky for even the most experienced industry veteran to understand. Just one misstep could mean significant and lasting consequences for your business. At Regulatory Compliance Associates, we offer the pharma consulting experience and pharma consultants necessary to guide you through the quality compliance process.
- Assessments
- Audits
- Regulatory Agency Response
- Preparation and Training
- Inspection Readiness
- Data Integrity
Quality Assurance
Regulatory Compliance Associates Quality consulting includes assessments, strategy, implementations, staff augmentations, and identification of quality metrics to ensure continuous improvement. Our pharma consultants understand the strategic thinking needed to align your business needs and goals. Regulatory Compliance Associates quality assurance services include quality experts with experience spanning major corporations and start-ups. Our pharmaceutical consulting firm knows firsthand how to achieve, maintain, and improve quality, and we excel in transferring pharma consulting knowledge to your organization.
- 21 CFR Part 11
- Data Integrity
- Manufacturing Support
- Facility Support
- Quality Metrics
Remediation Services
Regulatory Compliance Associates has a proven remediation services approach to managing FDA Warning Letters, Consent Decrees, Remediation and other serious regulatory situations. Our pharma consultants know how to partner with executive, legal, and communication teams. Each RCA pharma consulting Expert will develop a response that will be accepted by the regulatory agency and be realistic to execute.
Regulatory Compliance Associates pharma regulatory consultants will develop a comprehensive proof book of documented evidence demonstrating the corrective action taken to remediate non-compliant issues. In addition, each Regulatory Compliance Associates pharma consulting Expert understands compliance enforcement. We’ll prepare a comprehensive pharma consulting strategy to assist in your remediation efforts, drive continuous improvement, and maintain regulatory compliance with the regulations.
- Regulatory Action
- Regulatory Compliance
- Regulatory Enforcement
- Warning Letter
- 483 Observation
- Oversight Services
- Risk Management Plan
About Regulatory Compliance Associates
 Regulatory Compliance Associates® (RCA) provides pharmaceutical consulting to the following industries for resolution of life science challenges:
Regulatory Compliance Associates® (RCA) provides pharmaceutical consulting to the following industries for resolution of life science challenges:
- Life Sciences
- Pharmaceutical
- Biologic & Biotechnology
- Sterile compounding
- Medical device
- Lab Testing
We understand the complexities of running a life science business and possess areas of expertise that include every facet of R&D, operations, regulatory affairs, quality, and manufacturing. We are used to working on the front lines and thriving in the scrutiny of FDA, Health Canada, MHRA and globally-regulated companies.
As your partners, we can negotiate the potential minefield of regulatory compliance and regulatory due diligence with insight, hindsight, and the clear advantage of our unique expertise and experience.
- Founded in 2000
- Headquartered in Wisconsin (USA)
- Expertise backed by over 500 industry subject matter experts
- Acquired by Sotera Health in 2021
About Sotera Health
The name Sotera Health was inspired by Soteria, the Greek goddess of safety, and reflects the Company’s unwavering commitment to its mission, Safeguarding Global Health®.
Sotera Health Company, along with its three best-in-class businesses – Sterigenics®, Nordion® and Nelson Labs®, is a leading global provider of mission-critical end-to-end sterilization solutions and lab testing and advisory services for the healthcare industry. With a combined tenure across our businesses of nearly 200 years and our industry-recognized scientific and technological expertise, we help to ensure the safety of over 190 million patients and healthcare practitioners around the world every year.
We are a trusted partner to 5,800+ customers in over 50 countries, including 40 of the top 50 medical device companies and 9 of the top 10 pharmaceutical companies.
Commitment to Quality
Our Certificate of Registration demonstrates that our Quality Management System meets the requirements of ISO 9001:2015, an internationally recognized standard of quality.
To begin the Regulatory Compliance Associates scoping process today, please enter your information in the blue form below and click the submit button at the bottom of the webpage.


