Insights on driving value from staffing, automation and continuous improvement.
Developing a quality management system is the foundation for ensuring the organization’s products or services are safe, effective and controlled to deliver customer satisfaction. Throughout the organization’s lifecycle, from start-up through maturity, the quality needs of the firm, along with its budget constraints, are continually evolving. Maintaining compliance with regulations while controlling costs represents a challenging balancing act we encounter in our life science consultancy. The most successful firms apply critical assessment of their needs and gaps at present and in the future, and deploy a risk-based approach to their quality system.
Upfront Planning Avoids Costly Surprises
Before establishing a quality system, it’s critical to identify the quality standards, regulations and/or requirements surrounding the company’s products, service and business needs. The checklist in Table 1 provides some common areas to consider.
Gap Analysis
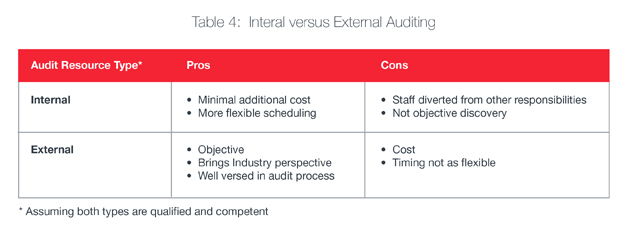
If your organization already has a quality system, perform a gap analysis against your identified standards/requirements per Table 1. If your quality team could benefit from an external assessment, consider hiring an experienced consultant or firm to provide expertise in the gap analysis.
ISO13485
If your organization is or will be manufacturing or distributing internationally, global considerations will add complexity to your quality system. For example, the following considerations can greatly impact the complexity of the quality system; European Union, ISO13485, Canadian Medical Devices Regulations, Brazilian GMP, and Japanese Ordinance #169, and others.
Phased Enhancements: Spread the Spend Over Time
Maximizing Value from Staffing
- Quality System Leader The organization requires a competent and knowledgeable individual as the quality system leader(s) who may implement the QMS, keep the system compliant, and maintain the design history file.
- Based on the lifecycle of the organization, the quality system leader may be tasked with implementing the QMS or just maintaining the QMS going forward. This could be two different skill sets.
- Tip: Early stage companies can benefit from outsourcing development of the QMS to highly experienced quality consultants and then transition maintenance of the QMS to less expensive in-house personnel.
- Skill Assessment By analyzing the skill sets of in-house quality personnel against needs, firms can identify gaps and develop solutions. A few considerations:
- Tip: Can current staff be trained? Training represents an upfront cost but offers long- term payoff.
Training Programs
- Utilize assessments and audits to understand the capabilities ceiling of your existing team. If training cannot close the critical gaps, consider whether the needed skill set is temporary or long-term. Temporary gaps can be closed cost effectively through hiring of contractors or DHF consultants to provide specialized knowledge without paying for a full-time employee. Such expertise can be used for:
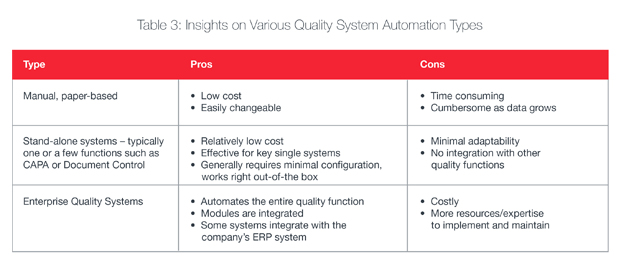
Maximizing Value from Automation
- Start-up or early stage organizations can benefit from basic manual systems or hybrid systems that automate some of the more labor-intensive quality functions such as document control.
- Mid-market organizations can benefit from increased automation through hybrid or enterprise QMS systems that address multiple quality needs such as document control, deviations control, non-conformance, equipment calibration, equipment maintenance orders, audit, CAPA, change control, training and functions that control product outputs.
- Large organizations can benefit from enterprise QMS systems and integrating those systems with their ERP systems for even greater interoperability.
Maximizing Value from Continuous Improvement
- Internal and external audits program
- Complaints
- Customer feedback
- CAPA system
- Product or process non-conformances
- Processing data
- Enforcement activity such as a FDA 483 or Warning Letter
- Costs relative to the budget
- Concerns about cybersecurity and cost
Budget Maximizing Tips
Travel:
- Limiting travel by employees and choosing local contractors or consultants to minimize this expense.
- For organizations with multiple locations, consider leveraging the geography of the company’s quality personnel. For example, if facility A has an out-of-state supplier located near sister facility B, then facility A might be able to tap into the facility B personnel to perform the supplier audit. The travel savings could offset the cost to quality facility B personnel on the QMS audit.
Training:
- Delaying training can minimize this expense. Although it can be less expensive in the long run to raise the skill set of your employees in areas such as internal audits, some companies trim budgets by foregoing to deferring training.
- Virtual cGMP training is another possibility to minimize training expense. By training all your employees at once, companies can negotiate reduced training fees and may possibly eliminate department travel by having a trainer come onsite to the company.
- Delaying supplier audits Sometimes audits can be scheduled to the next quarter or early next year to save expense based on the acceptable risk exposure.
- Group input: The quality team is the cornerstone of the QMS. Engaging these internal experts can identify creative opportunities to trim expenses while minimizing risk.
Today’s Quality leaders are responsible for managing the organization’s compliance and cost of quality, while driving customer satisfaction and maximizing their department budgets. Careful planning and judicious use of human capital and other resources are critical in deploying a risk-based strategy that delivers organizational results.
To begin the Regulatory Compliance Associates® scoping process today, please enter your information in the blue form below and click the submit button at the bottom of the webpage.