Life science companies understand the FDA inspection process is a cornerstone towards ensuring safety and efficacy in products. When companies conduct an internal audit and are prepared for inspections, it is easier for the FDA personnel to do their jobs of assessing compliance. Being prepared for inspections is not about cutting corners or feigning compliance, rather it is about everyone doing their part to ensure safety and efficacy. Patient safety is the top priority.
True inspection preparedness supplements internal audits with a practiced inspection process and behavioral training for key personnel.
Inspection Preparedness
Despite this knowledge, many organizations get bogged down in the day-to-day or get consumed by special projects. This results in inspection preparedness taking a back seat. For many medical device, biotech and pharmaceutical companies, inspection readiness becomes simply the internal audit program.
While internal audits are a key component of inspection preparedness, they tend to be informal, less intense and focused more on auditing than inspecting. True ISO audit inspection preparedness includes a practiced inspection process and audit training for employees.
Inspection Process
Before an FDA inspection occurs, organizations should have an inspection process and practice through mock inspections. Like any process, inspection readiness needs a plan that is executed, assessed and continuously improved upon.
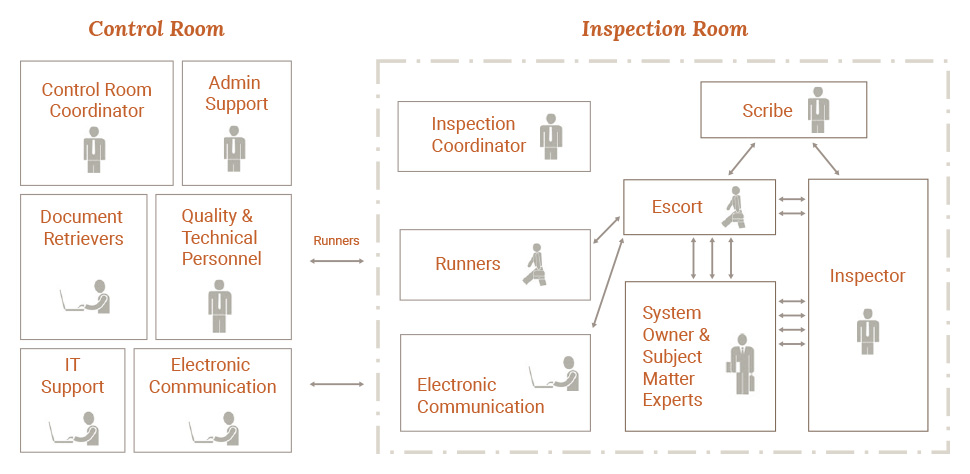
Ideally, the inspection process will encompass the activities of the Inspection Support Team. Process managers will assign employees to the Control Room. System Owners will collaborate on requests with the functional subject matter experts (SMEs).
Inspection Support Team
The Inspection Support Team is the primary interface with the FDA inspector. Team roles for audit control include the Inspection Coordinator, System Owners (or SMEs), the Escort and the Scribe. See below for an overview of the roles and responsibilities during an FDA inspection.
Inspection Room (Front Room)
The Inspection Coordinator manages the system of internal control and overall process in the Inspection Room. While inspectors often walk the facility, the Inspection Room provides a focal point for the FDA inspection. It is where questions are asked and answers are presented to inspectors. Moreover, this room is sometimes referred to as the Front Room where access to documentation should be readily available.
The Escort is the primary point of contact for the FDA inspector. This role manages the conversation with the Agency and completes audit data requests. These Front Room requests are given to a runner who then delivers to team members inside the Control Room. Further, the Control Room is commonly referred to as the Back Room of an inspection.
Control Room (Back Room)
After receiving the internal audit process request, the Control Room responds by either accessing documentation or setting up interviews with appropriate personnel. Data moves electronically or physically back and forth between the Control Room and Inspection Room. Additionally, video can also help augment data sharing among both teams.
The System Owners (e.g. SME’s) review and present materials to the FDA inspector. This role also provides continuity and context for answers. After SME’s are dismissed from the Inspection Room, they will return to the Control Room for debriefing and note taking similar to GMP audits.
Conversation Scribe
The Scribe is present for internal audit reporting and general conversation notes that are ongoing in the Inspection Room. These notes aid the team during and after the inspection. They are useful in developing responses needed in the moment or if future FDA observations arise.
Instant messaging, which has become popular in ISO 9001 audit formats due to the pandemic, is an asset between the Front and Back Rooms. Using technology to communicate instantly increases the collaboration about the critical issues being discussed.
For example, imagine if documents were not being delivered in a timely manner to the inspector and she/he is becoming anxious. It is helpful for the Inspection Room to stay connected with the Control Room about each audit need and the expected delivery time-frame.
Control Room Coordinator
The Control Room provides the documentation, interviewees and preparation that is needed to support the inspection process. This Back Room team, led by the Control Room Coordinator, processes requests for information, retrieves documents from electronic systems and also triages documents as needed.
The control room is staffed with quality and technical personnel who log requests for information and alert department heads within the organization. The control room helps to anticipate and prepare for the next potential questions from the inspectors.
Document Requests
The control room staff accesses and prepares the requested documentation for the SMEs. The SME process involves reviewing the retrieved documentation, preparing for a discussion with the inspector and then presenting in the Inspection Room.
SMEs are escorted to and from the Inspection Room for any last minute questions and to ensure debriefing occurs after interviews are completed.
Case Study
The Inspection and Control Room can scale up or down with the nature of the inspection. For example, one organization had FDA inspectors and other auditing bodies on-site for six months. Three Control Rooms were set up which had nearly 100 employees supporting the process and driving efficiencies in providing requested documentation.
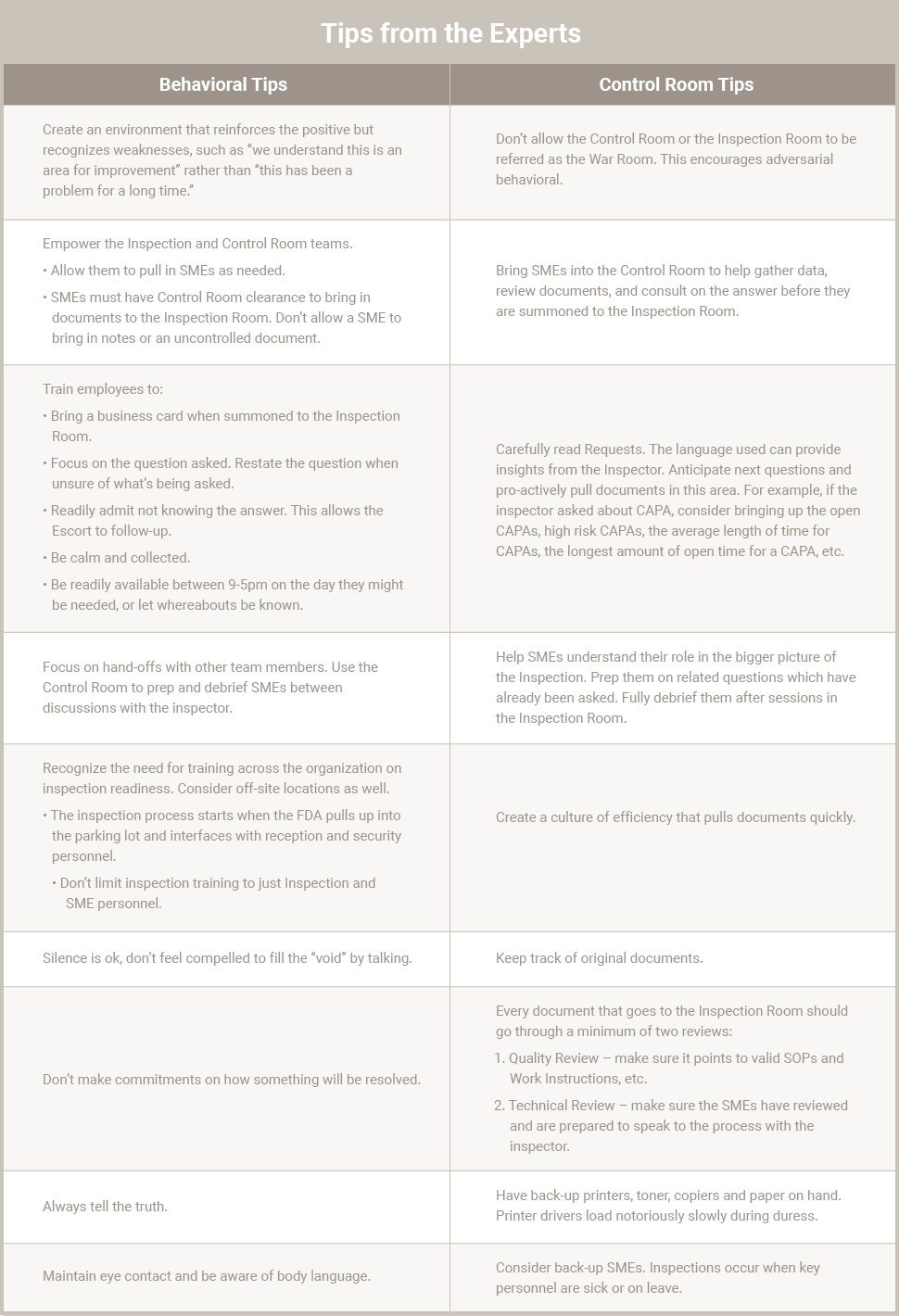
Behavioral Training
In years past, organizations were known to train ISO internal audit employees to also give curt answers to FDA inspectors in hopes that nothing more would be revealed externally to the agency. This approach created fear and mistrust among employees and set up adversarial relationships with the FDA staff.
Practicing the inspection process can be one of the best ways to help employees learn appropriate behaviors and in order to create the most beneficial inspection process environment, both the agency and the company need to form a partnership. Both the agency and industry want to provide safe and efficacious products, and both understand the role inspections play.
Mock Inspection
Many companies conduct a risk based audit as part of their inspection readiness. Mock inspections take the next step and allow companies to practice the Inspection Room, the Control Room and the right behaviors in working with inspectors to help them access information needed.
A mock inspection can be used to probe further on issues uncovered during internal audits. The mock Inspection Team should bring in SMEs and other personnel as needed for this activity. This helps the organization fully understand areas needing improvement and gives them a platform to take corrective action before the Agency actually arrives.
Too many organizations scramble when an FDA inspection occurs because they fail to be prepared. Since organizations don’t perform optimally on the fly, it’s important to prepare and practice the inspection strategy in advance, not simply rely on internal audits to ready the organization.
Summary
Best practice organizations understand inspection preparedness is vital in working with the agency to ensure safe and efficacious products. Inspection readiness involves both internal audits and mock inspections.
During these mock inspections, organizations set up Inspection Rooms and Control Rooms to help teams learn how to readily access documentation which would be requested during an inspection.
Additionally, these sessions give employees an opportunity to demonstrate proper behavior and interviewing techniques when working with the agency. Being prepared ensures that the inspection process will run smoother, decreases the likelihood of observations and helps groom the organization and individuals for higher levels of future performance.
Hope is not a strategy.
To begin the Regulatory Compliance Associates scoping process today, please enter your information in the blue form below and click the submit button at the bottom of the webpage. You may also email us at [email protected].



 Regulatory Compliance Associates® (
Regulatory Compliance Associates® (

